 |
 |
||
|
|
|||
RESEARCHPathophysiology of pancreatitis | ||||
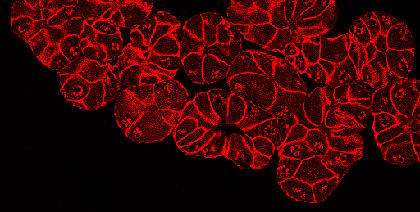
Pancreatitis is a debilitating inflammation of the pancreas, which in its severe form is associated with substantial morbidity and mortality and constitutes a major risk factors for developing pancreatic cancer. The etiologic factors involved in the initiation and aggravation of pancreatitis have not been completely identified, and a definitive treatment of the disease remains to be established. Recent studies show that pancreatic injury triggers a process of tissue regeneration, which is crucial for healing of the organ. We are particularly interested in understanding the molecular mechanisms governing regeneration of the injured pancreas, as a better knowledge of the events responsible for activation and proliferation of pancreatic acinar cells is likely to reveal new approaches for improving disease outcome. To this aim, we use experimental models of pancreatitis in genetically modified mice to determine the role of single molecules during the onset of the disease and in the phase of regeneration of the organ. | 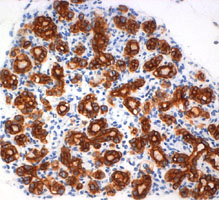 Formation of acinar to ductal metaplasia during pancreas regeneration increases in mice deficient for p21CIP1/WAF | |||
Molecular mechanisms of acinar cell secretion | ||||
Acinar cells of the pancreas play an essential role in the digestive process through the secretion of a variety of digestive enzymes. While the enzymes are typically secreted in an inactive form (zymogens) to prevent digestion of the pancreas, it is now well documented that aberrant secretion and premature activation of the zymogens cause cellular damage, which triggers the significant inflammatory response characteristic of pancreatitis. We are currently studying the cellular and molecular events controlling the secretory processes of pancreatic acinar cells, due to their crucial role in both physiological and pathological situations. Recently, by using biochemical approaches, molecular imaging and electron microscopy, we identified serotonin as an important regulator of actin-cytoskeleton dynamics which directly affects zymogen secretion in pancreatic acinar cells. | 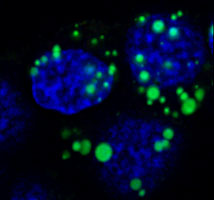 Zymogen amylase (green) accumulates in the secretory vesicles of acinar cells following inhibition of serotonin-dependent secretion | |||
Pancreatic cancer | ||||
Pancreatic ductal adenocarcinoma (PDAC) remains a devastating disease despite tremendous scientific efforts. Recent studies using sophisticated genetically engineered mice expressing mutated Kras, propose a stepwise process starting from acinar cells throughout premalignant PanIN (pancreatic intraepithelial neoplasia) lesions leading to tumor formation. Inflammation has been described as a risk factor in the development of PDAC, however cellular and molecular mechanisms of inflammation-driven pancreatic carcinogenesis so far remain elusive. Our primary goal is to understand how inflammatory cells contribute to the development of early premalignant lesions. Therefore we inter-crossed tg(ElaLT mice, which develop pancreatic inflammation, to the commonly used PTF1+/Cre; Kras+/G12D model for pancreatic cancer. We could show that the acinar specific over-expression of lymphotoxin in mice that harbour the Kras mutation dramatically accelerated the development of PanIn lesions. This model enables us to understand how inflammatory pathways interact in the pancreatic epithelium, which is a key step in the possible development of tools for early PDAC detection and treatment. |  Formation of PanIN lesions in KRasG12V mice accelerates following ectopic expression of lymphotoxin α, β in acinar cells | |||
Establishment of pancreatic stone protein (PSP) as a reliable clinical marker of sepsis | ||||
Sepsis is a life-threatening condition due to untreated or severe infections, leading to an uncontrollable inflammatory response throughout the body and eventually organ failure. Early detection of infection and development of sepsis is important to initiate adequate life-supporting therapy. Unfortunately, early markers of sepsis are not available or not reliable. Thus, there is an ongoing need for better diagnostic tests. In animal experiments, we observed that pancreatic stone protein (PSP), a protein produced by the pancreas during pancreatitis, is also markedly increased during inflammatory events affecting other organs. Therefore, we hypothesize that serum levels of PSP act as a biomarker for infection and sepsis. To test this hypothesis, we developed an ELISA assay able to accurately measure PSP levels in human serum and initiated several clinical trials to confirm the validity of this marker. PSP and REG1, a literature summary of known publications, by Oliver Galbier | ||||
©2018 The Pancreas Research Lab |